By Joseph C. Gentry
GTC Vorro Technology
Introduction
The refining industry is pressured. Environmental regulations dominate investment budgets, and often take the top priority in operating expenditures. Mandates on compliance rule over all else, and must be adhered as a license to operate regardless of the logic or cost/benefit analysis. The Gas Processing Industry faces some of the same challenges, and has found different ways to manage H2S that may be useful in the downstream refinery industry.
Redundancy in SRU
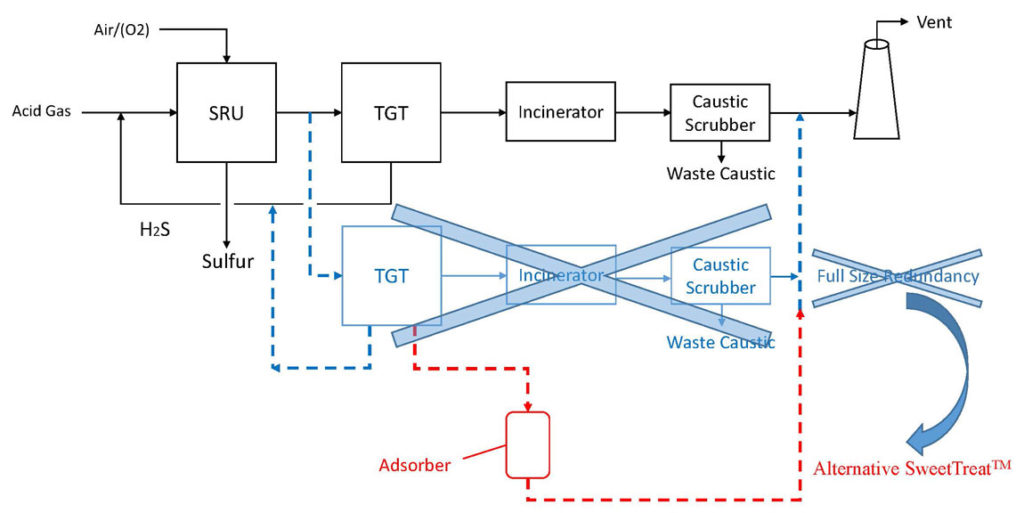
Refiners typically have a sulfur recovery unit (SRU) as catch-all for sulfur from the various processing units. Organic sulfur is removed from the oil in hydrodesulfurization units, where H2S is generated; then removed in an off gas. This gas is treated in an amine contacting unit to remove the H2S. The acid gas is then stripped and routed to the SRU, which uses the Claus technology to produce elemental sulfur. The SRU has long been a mainstay of the refinery operation; but now it is even more so required. New regulations require 100% redundancy of critical emissions control systems, including the sulfur recovery and tail gas treating (TGT) units. The goal is to completely eliminate flaring of the H2S gas during outages of the SRU and TGT, even during startup and shutdown1.
In times past, it was sufficient to have surplus capacity in the sulfur plant or multiple SRUs, in case one unit had to be taken offline. Now, there is need to coordinate the startup and shutdown sequences of the sulfur plant and the tail gas treating unit, which involve different types of unit operations and process dynamics. This requires a cumbersome coordination of process units just to make a normal startup or shutdown, which would otherwise require to temporarily flare a small amount of H2S gas.
One interesting option is to use a solid scavenger system, such as SweetTreatTM to remove the H2S during plant startup/shutdown, or during process upsets. This de-couples the main process units from emergency operation. The SweetTreatTM process is a passive, always-ready system to ensure that the acid gas is treated in every situation. The solid adsorbent converts 100% of the H2S into an iron-sulfide, which is suitable for disposal as non-hazardous waste. SweetTreatTM is a fixed vessel operation that is activated simply by opening valves to the unit. When the adsorbent is spent, it is dumped and re-loaded for the next incident. This is far better than keeping a full-size redundant amine and incinerator within the TGT unit ready on hot standby, or a caustic scrubber that requires disposal of spent caustic.
Energy Efficiency By Burning Sulfur-free Gas
The SRU with TGT units are designed for certain sulfur recovery, to meet an overall site allowance of SO2 emissions. The refinery-generated fuel gas contains some amount of H2S that converts mainly to SO2; but a small portion is further oxidized to SO3, which condenses with water to form H2SO4. To avoid the corrosive effects of sulfuric acid on the process equipment, the furnace is operated to keep the flue gas at a temperature level above the acid-gas dew point. This limits the furnace efficiency, which would otherwise maximize the extraction of useful heat and result in a lower flue gas temperature. If the refinery fuel gas had zero H2S, then the acid-gas dew point condition would be substantially lower, giving flexibility to optimize the furnace operation.
Let’s look at an example:
| Refinery fuel gas H2S content | 100 wppm (as set by recovery in the amine absorbers) |
| Resultant flue gas, SOx | 180 wppm |
| Resultant flue gas, SO3 | 5.6 wppm (assumes 3% is further oxidized) |
| Acid-Gas Dew Point | 130 DegC |

To guard against sulfuric acid being formed upon condensing the SO3 with water, the stack outlet temperature would normally be kept at 150 -160 DegC or higher. This precludes use of low-level heat recovery into combustion air or boiler feed water2,3. However, if the acid-gas dew point is not governing, the stack gases can be further cooled to the H2O dew point or lower (subject to reasonable temperature approach), thereby increasing the furnace efficiency4.
| Refinery fuel gas H2S conten | 0.1 ppm (with H2S removal by SweetTreatTM) |
| Resultant flue gas, SOx | 0.2 ppm |
| Resultant flue gas, SO3 | 0 |
| Acid-Gas Dew Point | < 100 DegC |
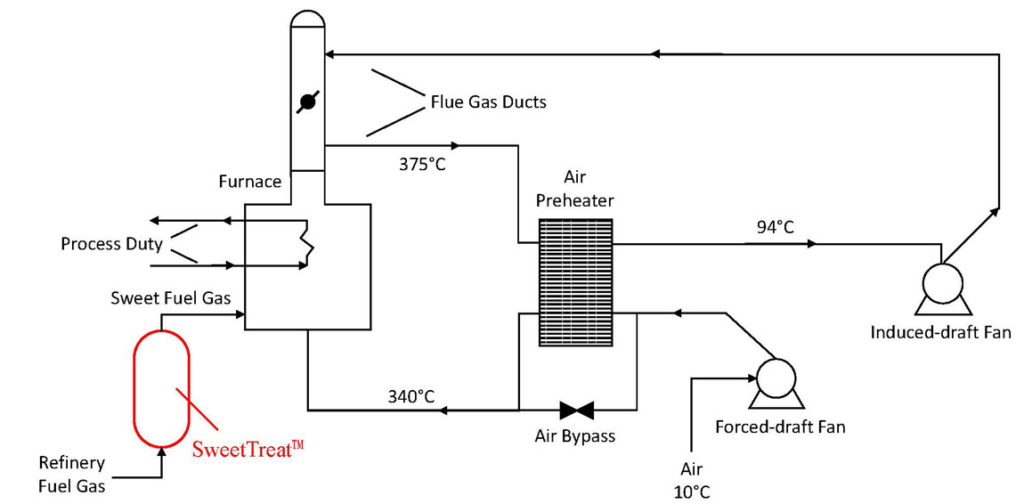

By availing of the full air preheating potential, there may be energy savings of 10-15% in the furnace. Higher combustion air temperature also makes it easier to control the level of excess O2, to reduce this to the minimum level. The energy savings comes with reduction in CO2 emissions from the refinery process heating, which can be useful to add to the refinery ESG scorecard7.
Even if the normal furnace operation maintains the stack temperature above the acid gas dew point, there can be times when the temperature may drop due to sudden weather change or turndown operation, bringing the system into the severe corrosion regime. Thus, it is important to avoid having H2S in the fuel system in any case. Sulfur is not our friend.
Another example:
Many refinery gas systems operate at low pressure. In order to get a satisfactory removal of H2S, they may use a strong, unhindered amine such as MEA. There are two issues with MEA which make it less efficient than hindered amines such as MDEA: 1) MEA holds the H2S more strongly, and requires more energy for regeneration. The MEA also absorbs most of the CO2, which requires regeneration cost. 2) CO2 in the acid gas would be recycled back to the Claus unit and TGT units, which incurs operating costs that would not be present if CO2 were excluded.
If the SweetTreatTM system were applied to the fuel gas, the refiner could relax the requirement for H2S removal in the amine system and use a lower-cost system for bulk H2S removal. SweetTreatTMremoves the final quantity of H2S in the fuel gas from the relaxed operation; nearly all of the CO2 can be rejected into the fuel gas away from the Claus and TGT units; and energy required to operate these units is reduced.
Sulfur Scavenging in Hydrogen Systems
The majority source of H2S in the refinery is from hydrodesulfurization units, which convert the organic sulfur into hydrogen sulfide. These units typically operate with hydrogen recycle gas to maintain the hydrogen partial pressure in the reactor. Some of the gas is purged to the fuel system, to maintain purity of the hydrogen and remove H2S. Depending on the quantity of sulfur in the liquid feed, there may be need to operate an amine absorber on the hydrogen recycle gas stream to remove the bulk of H2S. Even so, the amine will not capture all of the hydrogen sulfide, which returns to the reactor via the recycle hydrogen. H2S is generally a catalyst poison and should be removed as low as possible. The SweetTreatTM system can remove all of the H2S from the recycle hydrogen to improve the cycle length and operability of the hydrotreater.
One example is in the area of FCC gasoline selective hydrotreating. Here the objective is to remove the organic sulfur while avoiding to saturate the olefins. The ∆ RON between an olefin and its corresponding paraffin is about negative 50! If H2S remains in the recycle hydrogen, it will recombine with olefins to create new mercaptan sulfur species, defeating the purpose of the hydrotreatment and requiring higher severity. Additionally, H2S poisons the catalyst. Thus, it is important to eliminate all H2S from the hydrogen gas.
We can apply the SweetTreatTM process within the hydrogen recycle loop to greatly improve the functional efficiency of the selective gasoline hydrotreatment system. This fixed bed adsorption system is installed on the recycle loop itself, or after the amine absorption unit if the sulfur content is extremely high. In either case the octane retention is improved, hydrogen consumption lowered, and operating costs outside the HDS unit are reduced.

Claus and TGT Improvement
The first area to get attention for sulfur reduction was the Claus unit off gas, to add tail gas treating (TGT). One of the well-known options is the SCOT8 unit offered by Shell. The SCOT consists of a hydrogenation step to convert all of the sulfur species into H2S, which is then re-absorbed into an amine for recycle to the sulfur recovery unit. In some cases, the remaining off-gas from the amine absorber may be sent to thermal oxidizer (TO) to convert all residual H2S into SO2, then absorbed with caustic wash to yield a final product suitable for venting. This is a complex arrangement to get the last traces of hydrogen sulfide removed from the vent.
One improvement that can be made to this arrangement is to use the SweetTreatTM process as replacement of the thermal oxidizer and caustic wash. This will eliminate the energy required in the TO, and the waste caustic to be handled. Alternatively, the SweetTreatTM process can be operated as a back-up to the amine and caustic wash / T.O. part of the TGT unit, if these needs to be shut down for a short duration.
A further enhancement would be to use a weaker amine in the TGT absorber. This permits easier and less costly regeneration of the H2S, and could further reduce the CO2 that is captured and recycled to the Claus plant. Di-isopropyl amine (DIPA) for example, may take 0.10-0.15 moles of CO2 with each mole of H2S that is recycled to the Claus reaction section. Optimizing the amine choice and operating conditions may drop the CO2 capture to 5% or less. Any elevated content of H2S in the resulting off-gas would be removed in the SweetTreatTM adsorber, similar to the redundancy application of Fig. 1.
Zero Sulfur Refiner
The logical progression of environmental activism is to have zero emission of whatever is offending. ESG initiatives provide the impetus to take action on reducing noxious waste to the atmosphere. In the case of sulfur, there is a pathway to get to zero sulfur leaving the refinery in the atmospheric emissions by removing all H2S from the flare gas, TGT off-gas, and fuel gas streams. Traditional unit operations leave some small quantity of H2S, which may require further treatment in an incinerator or caustic scrubbing system. SweetTreatSM is a solid adsorbent system that eliminates all of the H2S in these streams.
SweetTreatSM can be used in an emergency or standby mode linked with the flare system; and used on a continuous basis on the refinery fuel gas and off gases. This is a way to boost public perception and community good, by going below the requirements set by a site limit on SOx emissions.

Conclusion
Sulfur is objectionable on account of equipment corrosion and acid rain, and general toxicity of H2S and SO2. Environmental regulations are squeezing every aspect of refinery emissions, including sulfur. Thus, refiners should avail of the best options to reduce sulfur emissions. The Gas Processing Industry extensively uses solid adsorption systems as scavengers for H2S. Similar systems can also apply to its cousins in the downstream refining industry.
- Solid adsorption scavenger systems are excellent means to provide redundancy to the TGT and eliminate flaring. They give continuous and complete removal of sulfur.
- Removing all H2S from the fuel gas allows the furnaces to operate with a lower stack temperature, extracting more heat from the fuel gas. Overall there could be an increase of 3-4% in refinery energy efficiency by recovery of the low-level heat from the fired heaters.
- Scavenging sulfur in the hydroprocessing units reduces hydrogen consumption and improves operating performance such as gasoline octane value.
- Claus/TGT and amine optimization can be made by judicious use of fixed-bed scavenger system at key points in the process. This will reduce emissions going to the flare and directionally debottleneck the sulfur processing units.
A ‘Zero Sulfur Refiner’ will improve its ESG score, and be recognized as a leader that is sensitive to environmental issues.
References
- Crowther, David, presentation at 2021 GPA Midstream Conference
- Pim Van Keep, Robert Sakko, PTQ Q2, 2019.
- Robert Torgerson, Gayesco publication, 11/10/2020
- Byron Eslinger, Tulsa Heaters, Inc., consultant note, Dec. 8, 2021
- SRI Intl. Report 29C; original ref Oil & Gas Journal, Sept. 2, 1974, p. 59-67
- SRI Intl. Report 29C; original reference Dittman H., et. al; Linde Ber Tech. Wiss., 51, 1982
- S. Singh and R. Mukherjee, Hydrocarbon Processing, October 2021.
- SCOT is licensed by Shell Catalysts & Technologies

